
(Get Answer) C 2 H4 Mg(HCO3)2 AlBr3 NO Which Of The Given Formulas Represent... Transtutors
Magnesium bicarbonate PubChem CID 102204 Structure Molecular Formula Mg(HCO3)2 C2H2MgO6 Synonyms Magnesium bicarbonate 2090-64-4 Magnesium bis (hydrogen carbonate) magnesium hydrogen carbonate magnesium;hydrogen carbonate View More. Molecular Weight 146.34 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound

Which of the following statements regarding the property of hard water is/are correct?1
Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3+ +3 magnesium Mg 2+ +2 carbonate CO 3 2- -2 ammonium NH 4 + +1 manganese (II) Mn 2+ +2 chlorate ClO 3 - -1 barium Ba 2+ +2 manganese (III) Mn 3+ +3 chloride Cl - -1 cadmium Cd 2+ +2 mercury (I)

Write the chemical name of the Mg(HCO3)2
Steps to calculate molar mass Identify the compound: write down the chemical formula of the compound. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Find atomic masses: look up the atomic masses of each element present in the compound.

Temporary hardness of water is due to the presence of
Magnesium bicarbonate or magnesium hydrogencarbonate, Mg ( H CO 3) 2, is the bicarbonate salt of magnesium. It can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia). It can be prepared through the synthesis of magnesium acetate and sodium bicarbonate :

Solution Name of the hardness Amount of Physical Chemistry
Question: Name the following ionic compounds. (a) Fe(OH)2 (b) FeSO3 (c) Mg(HCO3)2 (d) NaCIO (e) Ca(C2H302)2 (f) Sc203 (g) Ag2Cr207 (h) Mg(CIO3)2 (i) Co(NO3)2 ) (NH4)2Cr207 . Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We reviewed their content and use your.

How to Write the Name for Mg(HCO3)2 YouTube
The systematic name of Mg (HCO3)2 is magnesium carbonate O magnesium bicarbonate magnesium formate O magnesium acetate 111 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

In this video, we are going to share the process for naming the polyatomic chemical compounds.
Q1 What is the difference between magnesium carbonate and magnesium bicarbonate? Ans: The chemical formula of magnesium carbonate is MgCO 3 and magnesium bicarbonate is Mg (HCO 3) 2 . The magnesium carbonate is an inorganic salt, a white solid. Whereas magnesium bicarbonate exists in an aqueous solution. Q2

(i) Boiling During bour, g, the soluble Mg(HCO3 )2 is converted int in...
The first step to finding the molar mass of Magnesium Bicarbonate is to count the number of each atom present in a single molecule using the chemical formula, Mg (HCO3)2: 2. Find Atomic Mass of Each Element Next, using the periodic table, find the atomic mass in g/mol of each element (the molar mass of an element is equal to its atomic mass): 3.

What is the correct increasing order of solubility of the following salt? NaHCO3, KHCO3, Mg(HCO3
Click here👆to get an answer to your question ️ Write chemical names of the following compound: Mg(HCO3)2

How to find the molecular mass of Mg(HCO3)2 (Magnesium Bicarbonate) YouTube
a. FeCl3 iron chloride b. NO2, nitrogen (IV) oxide c. CaO, calcium (II) monoxide d. Al2S3, dialuminum trisulfide e. Mg (C2,H3O2)2, manganese diacetate f. FePO4, iron (II) phosphide g. P2S5, phosphorus sulfide h.
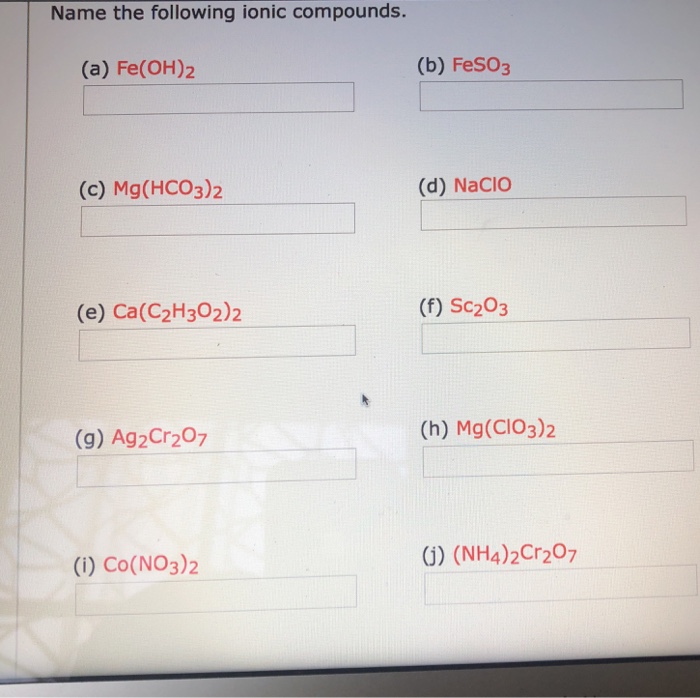
Solved Name the following ionic compounds. (a) Fe(OH)2 (b)
Magnesium Bicarbonate. Magnesium bicarbonate, also known by its IUPAC name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula C 2 H 2 MgO 6 or Mg (HCO 3) 2 [1]. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2].

How to write name for Mg(HCO3)2 YouTube
Write the names of the following compounds and identify each as ionic or covalent compound: Mg(HCO3)2 _____ CO _____ P4 _____ SiS2 _____ PBr3 CHAPTER 6: Compounds and their Bonds 1. Write the names of the following compounds and identify each as ionic or covalent compound:
Mg Oh 2 Hcl Compuesto
From a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. Mr. Montgomery

SOLVED A sample of water on analysis has been found to contain the following in ppm Ca(HCO3)2
Calculate the molecular mass of the following:Mg(HCO3)2 (Magnesium Bicarbonate)SUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34W.

Nêu kết quả sau khi trộn lẫn 5 dung dịch NaHSO4, NaCl, Mg(HCO3)2, Na2CO3, Ba(HCO3)2 với nhau
Naming Acid Salts 5.0 (1 review) Get a hint Mg (H2PO3)2 Click the card to flip 👆 magnesium dihydrogen phosphite Click the card to flip 👆 1 / 14 Flashcards Learn Test Match Q-Chat Created by chelsea_batchelor9 Students also viewed BIO objective 3 10 terms luvbubba Preview Naming Acid Salts Teacher 26 terms amanda_wichterman Preview
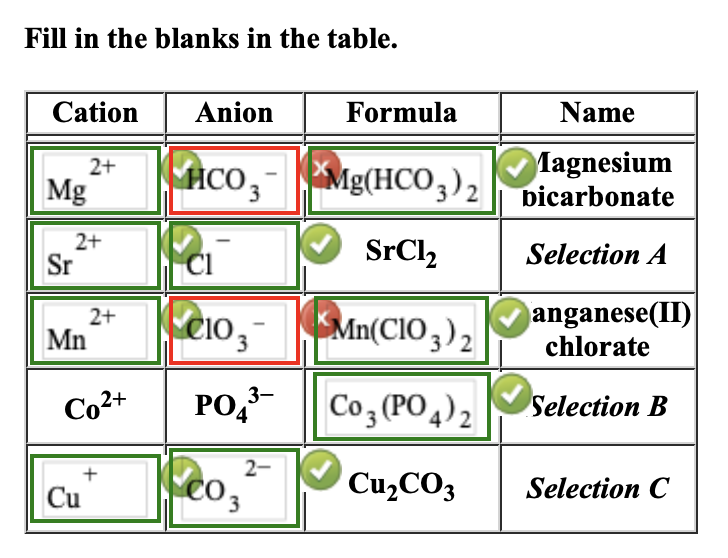
Solved Fill in the blanks in the table. Cation Anion Formula
Magnesium Hydrogen Carbonate Name: Magnesium Hydrogen Carbonate Alias: Magnesium Bicarbonate Formula: Mg (HCO3)2 Molar Mass: 146.3387 Mg (HCO3)2 Molar Mass Converter Weight: Mole: Mg (HCO3)2 is a white powder at room temperature. It is soluble in water. If the concentration of Mg (HCO3)2 or Ca (HCO3)2 is too high, the water is called hard water.